
The grasses are the most important plant family for food production. Despite the domestication of Oryza sativa L. (rice), Triticum aestivum L. (wheat), Zea mays L. (corn), Hordeum vulgare L.(barely), Secale cereale L. (rye), Avena sativa L. (oats), Sorghum bicolor (L.) Moench (sorghum), Saacharum officinarum L. (sugar cane), Pennisetum glaucum (L.) R. Br. (pearl millet), Panicum virgatum L. (switchgrass), Eleusine coracana (L.) Gaertn (finger millet.) and Eragrostis tef (Zucc.) Trotter (tef), the family has not been widely studied biogeographically (Bouchenak-Khelladi et al., 2010). Other notable economic uses of grasses include landscaping, construction (primarily bamboos), and biofuel production (Miscanthus x giganteus J.M. Greef & Deuter ex Hodk. & Renvoize, Panicum L., and Zea L.).
The grass family was probably characterized as a distinct entity in many cultures, and the first scientific subdivision of the family was made by Brown (1814), who recognized two different spikelet types between Panicoideae and Pooideae. A synapomorphy for the family is a one-seeded indehiscent fruit (seed coat is fused with the ovary wall), known as a caryopsis or grain (Peterson, 2013). Although in some Chloridoideae and bamboos the seed coat can be free from the pericarp and therefore, technically, is an achene. The grain is rich in endosperm starch, although it contains some protein and small quantities of fats (lipids). The embryo is located on the dorsal side of basal portion of the caryopsis and contains high levels of protein, fats, and vitamins. The ventral side is often sulcate and is marked by a hilar scar that may be basal and small or extend as far as the apex. The stems are referred to as culms and the roots are fibrous, principally adventitious arising from lower portions of the culms. Silica is a conspicuous component of the vegetative epidermis and stored in silica cells. Many grasses have rhizomes (underground stems) or stolons (horizontal above-ground branches) that allow for vegetative reproduction in perennial grasses. Another important feature of grasses is intercalary meristems, which allow growth well below the culm apex, typically near the base of the plant. The leaves are parallel-veined and two-ranked, with the basal portion forming cylindrical sheaths and the upper portions referred to as a blade. A ligule, located on the upper surface at the junction of the blade and sheath, commonly consists of flaps of tissue or hairs. The primary inflorescence is referred to as a spikelet with 1–many, two ranked bracts inserted along the floral axis or rachilla. The lowest two bracts of each spikelet, inserted opposite each other, are called glumes, above which, along the rachilla (axis), are borne paired bracts termed florets. Each floret consists of a lemma (lower bract) and palea (upper bract). Within each palea the highly reduced flowers can be found. Each grass flower usually consists of two or three small scales at the base called lodicules, an ovary with a style and usually two plumose stigmas and 1–6 but more commonly 3 stamens with basifixed anthers that contain single-pored pollen grains. Lodicules function to open the florets during flowering and represent reduced perianth (sepals and petals) segments (Yadav et al., 2007).
The possession of small pollen grains well adapted for wind aided dispersal and intercalary meristems allowing culms to resprout near the base after repeated episodes of fire and/or grazing has enabled the family to be extremely successful in planet-wide radiation and colonization, and grasses are often found in open and frequently disturbed habitats. Two major photosynthetic CO2 assimilation pathways (C3 and C4) are found in the grasses, and there are anatomical, physiological, phytogeographical, and ecological differences between these two types. C3 grasses are well adapted to temperate climates with winter precipitation, whereas C4 grasses are well suited to tropical and desert environments with summer/autumn precipitation. The addition of C4 photosynthesis has allowed the grasses to outcompete other plants in warm, tropical environments by lowering the oxidation levels (photorespiration) of photosynthetic products. These features have led to the family′s ability to occupy 31%–43% of the Earth′s surface in various climatic environments as the dominant component, the grasslands (Gibson, 2009).
The study of biogeography has blossomed into a major discipline of evolutionary biology (Funk et al., 2009; Wen et al., 2013; Wen & Wagner, 2020) and for this special issue we present the current knowledge on the derivation of major lineages that reside in the grass family (also see Welker et al., 2020). Using the fossil record to aid in dating our phylogenetic trees and combining molecular sequence studies of DNA, we reconstruct ancestral states to infer the origin of major clades, i.e., subfamilies, tribes, supersubtribes, subtribes, and selected genera in the grasses. For this special issue, we invited a broadly trained group of 35 scientists to collaborate and present their most recent advances in the biogeography and phylogenetics within the grasses.
Four of the manuscripts in this issue include new dated biogeographical analyses (Gallaher et al., 2022; Peterson et al., 2022b; Soreng et al., 2022a; Zhou et al., 2022); three comprise new phylogenetic analyses with subsequent taxonomic novelties (Da Silva et al., 2022; Peterson et al., 2022a, 2022c), one is an ecological study investigating functional traits in a clade of savanna/wetland grasses (Arthan et al., 2022), one looked at the current distribution of the grasses over the Australian continent to determine migration directionality (Bryceson & Morgan, 2022), and one is an updated classification of the family primarily based on molecular phylogenetic studies (Soreng et al., 2022).
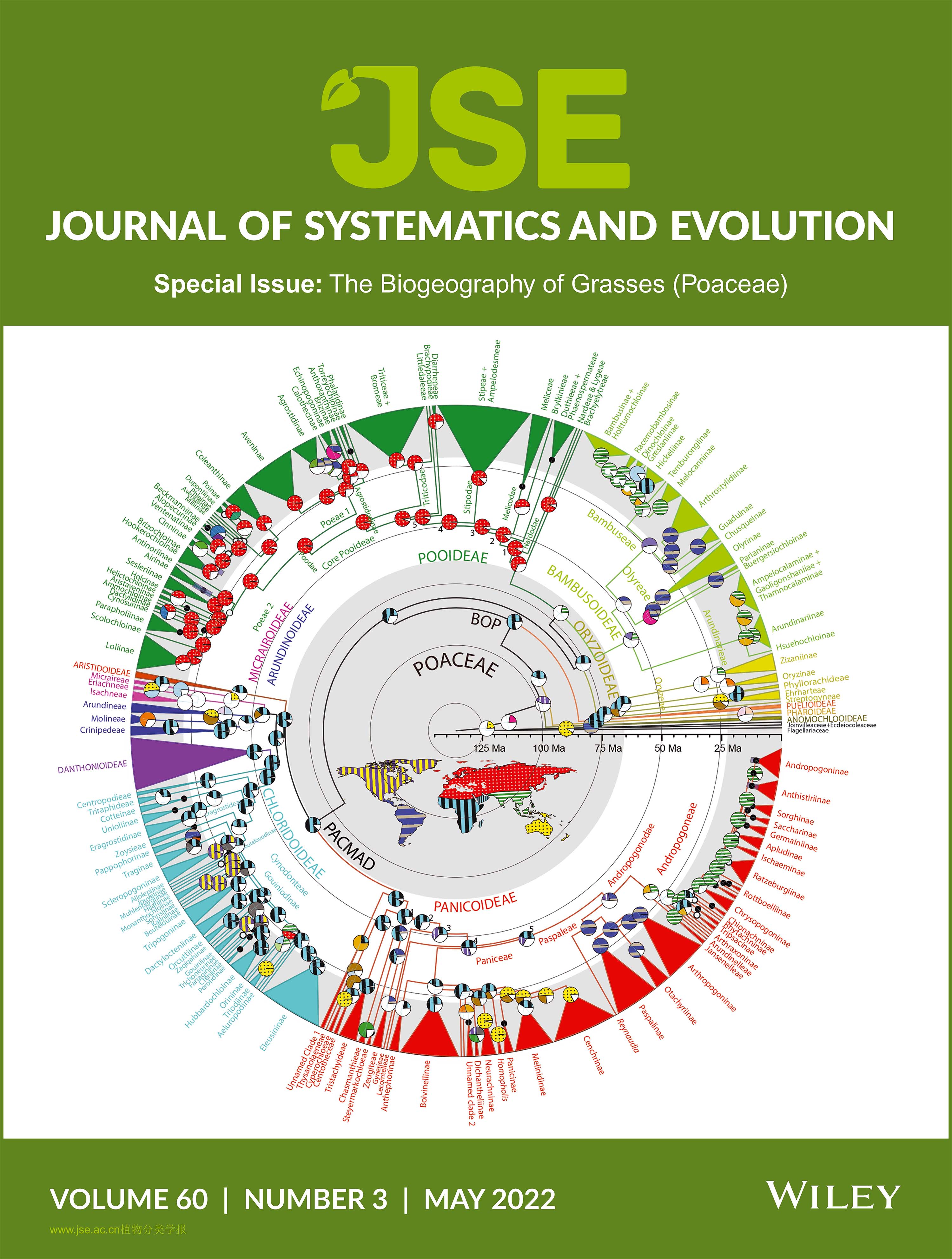
The Poaceae began to diversify in the early–late Cretaceous (crown age of 98.54 Ma) on West Gondwana before the complete split between Africa and South America, and Africa clearly served as the center of origin for much of the early diversification of the lineages within the family (Gallaher et al., 2022). In addition, Gallaher et al. (2022) includes an extensive review of the extant diversity and distribution of species, molecular and morphological evidence supporting the current classification scheme, and a biogeographical history of most lineages. Soreng et al. (2022a) found that the ancestral area of the Alopecurinae, Avenulinae, Coleanthinae, Miliinae, Phleinae, and Poinae (formerly referred to as PPAM clade) was southwestern Asia (including the Caucasus Mountains), originating in the early Miocene (crown mean of 21.81 mya). Muhlenbergia Schreb. apparently originated 9.3 mya in the Sierra Madre (Occidental and Oriental) in Mexico before splitting into six lineages, of which one (M. subg. Muhlenbergia) via long-distance dispersal, colonized Central Asia 1.6–1 mya (Peterson et al., 2021b). Melocanninae originated in the East Himalaya to northern Myanmar in the early Miocene (crown mean of 19.68 mya), and three routes were revealed in forming its present biogeographic pattern: in situ diversification on the Asian mainland, dispersing southwest to Sri Lanka and to the Western Ghats in South India, and spreading southeast to Malesia and Oceania via the Indo-China Peninsula (Zhou et al., 2022).
In their molecular analysis Da Silva et al. (2022) present evidence to support the polyphyly of Chascolytrum Desv. s.l. (Calothecinae), dividing it into nine genera: Boldrinia L.N. Silva, Calotheca Desv., Chascolytrum, Erianthecium Parodi, Lombardochloa B. Rosengurtt & B.R.Arrill., Microbriza Parodi ex Nicora & Rúgolo, Poidium Nees, Rhombolytrum Link, and Rosengurttia L.N. Silva; and describe a new subtribe, Paramochloinae L.N.Silva & Saarela, to include two genera, Laegaardia P.M. Peterson, Soreng, Romasch. & Barberá and Paramochloa P.M. Peterson, Soreng, Romasch. & Barberá. Peterson et al. (2022a), in a molecular study of nine Eleusininae genera, found Coelachyrum Hochst. & Nees polyphyletic and Schoenefeldia Kunth to be paraphyletic, subsequently describing a new genus, Schoenefeldiella P.M. Peterson with a single species and transferring Apochiton burttii C.E. Hubb. to Coelachyrum. In another molecular study, Peterson et al. (2022c) investigated Calamagrostis Adans. (Agrostidinae), hypothesizing the phylogenetic and biogeographical history of seven major species groups and proposing a new genus, Condilorachia P.M. Peterson, Romasch. & Soreng, while subsuming Dichelachne Endl. into Pentapogon R.Br. (Echinopogoninae).
Arthan et al. (2022) investigated the evolution of functional traits of 31 species in the Heteropogon-Themeda clade and found culm height, leaf length, leaf area, awn length, and awn types separated species occupying wetland and grassland/savannas; and that two widespread species, Heteropogon contortus (L.) P. Beauv. ex Roem. & Schult. (South American) and Themeda triandra Forssk. (African) have significantly different bioclimate niches. In their biogeographical survey of Australian grasses, Bryceson & Morgan (2022) suggest Southeast Asia was the gateway for largely one-way dispersal of C4 grasses into Australasia, and that the Paniceae were the earliest arrivals, followed by the Chloridoideae and the Andropogoneae. The worldwide phylogenetic classification in this issue includes 12 subfamilies, seven supertribes, 54 tribes, five supersubtribes, 109 subtribes, and an updated list of the number of species in each of the 787 accepted genera (Soreng et al., 2022b).
We thank Jun Wen for proposing the topic and supporting this special issue, and acknowledge all collaborators for contributing their work. We wish continued success to all agrostologists in uncovering mysteries in the evolutionary history of the grasses.
We present an updated worldwide phylogenetic classification of Poaceae with 11 783 species in 12 subfamilies, 7 supertribes, 54 tribes, 5 super subtribes, 109 subtribes, and 789 accepted genera. The subfamilies (in descending order based on the number of species) are Pooideae with 4126 species in 219 genera, 15 tribes, and 34 subtribes; Panicoideae with 3325 species in 242 genera, 14 tribes, and 24 subtribes; Bambusoideae with 1698 species in 136 genera, 3 tribes, and 19 subtribes; Chloridoideae with 1603 species in 121 genera, 5 tribes, and 30 subtribes; Aristidoideae with 367 species in three genera and one tribe; Danthonioideae with 292 species in 19 genera and 1 tribe; Micrairoideae with 192 species in nine genera and three tribes; Oryzoideae with 117 species in 19 genera, 4 tribes, and 2 subtribes; Arundinoideae with 36 species in 14 genera and 3 tribes; Pharoideae with 12 species in three genera and one tribe; Puelioideae with 11 species in two genera and two tribes; and the Anomochlooideae with four species in two genera and two tribes. Two new tribes and 22 new or resurrected subtribes are recognized. Forty-five new (28) and resurrected (17) genera are accepted, and 24 previously accepted genera are placed in synonymy. We also provide an updated list of all accepted genera including common synonyms, genus authors, number of species in each accepted genus, and subfamily affiliation. We propose Locajonoa, a new name and rank with a new combination, L. coerulescens. The following seven new combinations are made in Lorenzochloa: L. bomanii, L. henrardiana, L. mucronata, L. obtusa, L. orurensis, L. rigidiseta, and L. venusta.
Grasses are widespread on every continent and are found in all terrestrial biomes. The dominance and spread of grasses and grassland ecosystems have led to significant changes in Earth′s climate, geochemistry, and biodiversity. The abundance of DNA sequence data, particularly chloroplast sequences, and advances in placing grass fossils within the family allows for a reappraisal of the family′s origins, timing, and geographic spread and the factors that have promoted diversification. We reconstructed a time-calibrated grass phylogeny and inferred ancestral areas using chloroplast DNA sequences from nearly 90% of extant grass genera. With a few notable exceptions, the phylogeny is well resolved to the subtribal level. The family began to diversify in the Early–Late Cretaceous (crown age of 98.54 Ma) on West Gondwana before the complete split between Africa and South America. Vicariance from the splitting of Gondwana may be responsible for the initial divergence in the family. However, Africa clearly served as the center of origin for much of the early diversification of the family. With this phylogenetic, temporal, and spatial framework, we review the evolution and biogeography of the family with the aim to facilitate the testing of biogeographical hypotheses about its origins, evolutionary tempo, and diversification. The current classification of the family is discussed with an extensive review of the extant diversity and distribution of species, molecular and morphological evidence supporting the current classification scheme, and the evidence informing our understanding of the biogeographical history of the family.
To investigate the evolutionary relationships and biogeographical history among the species of Calamagrostis and other members of subtribes Agrostidinae, Calothecinae, Echinopogoninae, and Paramochloinae, we generated a phylogeny based on DNA sequences from one nuclear ribosomal (ITS) and three plastid regions (rpl32-trnL spacer, rps16-trnK spacer, and rps16 intron). Based on our phylogeny, we identified seven species groups (clades) within Calamagrostis: the Meridionalis group comprises two species from Central and South America, the Americana group comprises species from North America, the Deyeuxia and Epigeios groups comprise species from Eurasia, the Orientalis group comprises species from East Asia, the Purpurea group comprises species from Eurasia and North America, and the Calamagrostis group comprises species from Eurasia and North America. We hypothesize that Calamagrostis originated in North America with the primary split of the Meridionalis group, followed by split between the autochthonous Americana group and two future Eurasian branches encompassing all the remaining groups, which possibly dispersed into Eurasia independently. The molecular data suggest that hybridization and genomic introgression played a prominent role in the evolutionary history of Calamagrostis. We propose a new genus, Condilorachia, segregated from Trisetum s.l., with three species from South America for which we propose new combinations: Condilorachia bulbosa, Condilorachia brasiliensis, and Condilorachia juergensii; a new combination in Greeneochloa, Greeneochloa expansa; and the subsumption of Dichelachne into Pentapogon with 20 new combinations: Pentapogon avenoides, Pentapogon brassii, Pentapogon chaseianus, Pentapogon crinita, Pentapogon densus, Pentapogon frigidus, Pentapogon gunnianus, Pentapogon hirtella, Pentapogon inaequiglumis, Pentapogon lautumia, Pentapogon micrantha, Pentapogon parva, Pentapogon quadrisetus, Pentapogon rara, Pentapogon robusta, Pentapogon scaberulus, Pentapogon sclerophyllus, Pentapogon suizanensis, Pentapogon sieberiana, and Pentapogon validus. We provide a diagnosis, description, and a key to the species of Condilorachia.
We conducted a biogeographic analysis of the PPAM clade of Poeae Plastid DNA Group 2, which includes 12 subtribes of C3 grasses. One hundred and eighty-four species sampled represent 42 of 43 accepted genera and taxonomic diversity in large genera. We analyzed plastid sequences of matK, trnC-rpoB, and trnT-trnL-trnF using BEAST to produce a dated tree and MrBayes to produce a Bayesian tree, on which we ran Bayesian-Binary-Markov-Chain analyses on a worldwide biogeographic data set of 12 areas. PPAM split in southwestern Asia into subtribe Coleanthinae and PAM clades in the Early Miocene. PAM diversified rapidly in the Middle Miocene in southwestern Asia into four monogeneric lineages, Avenulinae, Phleinae, Miliinae, Poinae, and the Alopecurinae superclade (seven subtribes with 27 genera). In the Late Miocene, Pliocene, and mostly Pleistocene, the latter four lineages diversified and dispersed across Eurasia and established in North America. Dispersals to the southern hemisphere occurred in the Pliocene and Pleistocene. Annuals occur in 15 Mediterranean and southwestern Asia genera, but in few genera in other regions. Beyond phylogenetically isolated annual species dating to the Miocene, all other annuals evolved in the Pliocene and Pleistocene. Cold tolerance is high among perennial species, many occurring in the alpine, nine genera ranging into the Arctic. We suggest that alpine and subalpine habitats were ancestral. High tolerance of saline and alkaline conditions arose between the Pliocene and Pleistocene in Coleanthinae, Alopecurinae, Poinae, Hookerochloinae, Beckmanniinae, and Arctopoa. Combinations are proposed for Cornucopiae alopecuroides in Alopecurus and for Paracolpodium colchicum in Hyalopodium. A nothogenus × Catanellia is proposed for Catabrosa × Puccinellia.
Melocanninae is sister to other subtribes of Paleotropical woody bamboos with some 90 species mainly concentrated in Asia. However, phylogenetic relationships within the subtribe are poorly known. Here, we filled the gaps in complete plastome data of Melocanninae, reconstructed the phylogeny of Melocanninae, and further estimated divergence time and ancestral distribution range. Our results showed that the two major genera, Cephalostachyum Munro and Schizostachyum Nees, were paraphyletic. Species of Cephalostachyum were resolved in two successive basal clades, whereas Annamocalamus H. N. Nguyen, N. H. Xia, & V. T. Tran was embedded in the Schizostachyum clade. Different plastid regions provided inconsistent signals for the relationship of Melocanna and Pseudostachyum. Conservative loci supported a successive divergence rather than sister relationship between them and the difference may be caused by long-branch attraction. We infer that Melocanninae originated in the East Himalaya to northern Myanmar in the early Miocene. Three routes were revealed in forming its present biogeographic pattern: in situ diversification on the Asian mainland, dispersing southwest to Sri Lanka and to the Western Ghats in South India, and spreading southeast to Malesia and Oceania by way of the Indo-China Peninsula. The rapid uplift of the Tibetan Plateau and the intensification of Asian monsoons since the Miocene and the sea level fall events since the Late Miocene might be potential driving forces for diversification of Melocanninae and, particularly the latter event, for the species radiation of Schizostachyum.
Species of the Heteropogon-Themeda clade are ecologically important grasses distributed across the tropics, including widespread species, such as the pantropical Heteropogon contortus and Themeda triandra, and range-restricted species such as Heteropogon ritchiei and Themeda anathera. Here, we examine habitat preferences of the grassland/savanna and wetland species by describing bioclimatic niche characteristics, characterizing functional traits, and investigating the evolution of functional traits of 31 species in the Heteropogon-Themeda clade in relation to precipitation and temperature. The climatic limits of the clade are linked to mean annual precipitation and seasonality that also distinguish seven wetland species from 24 grassland/savanna species. Tests of niche equivalency highlighted the unique bioclimatic niche of the wetland species. However, climatic factors do not fully explain species geographic range, and other factors are likely to contribute to their distribution ranges. Trait analyses demonstrated that the wetland and grassland/savanna species were separated by culm height, leaf length, leaf area, awn length, and awn types. Phylogenetic analyses showed that the wetland species had tall stature with long and large leaves and lack of hygroscopic awns, which suggest selective pressures in the shift between savanna/grassland and wetland. The two most widespread species, H. contortus and T. triandra, have significantly different bioclimatic niches, but we also found that climatic niche alone does not explain the current geographic distributions of H. contortus and T. triandra. Our study provides a new understanding of the biogeography and evolutionary history of an ecologically important clade of C4 tropical grasses.
Australia's flora and fauna have long been considered unique, but whether this applies to its grasses is less known. This study characterises the Australasian grass flora biogeographically. We investigate the distribution of C3 and C4 grass genera across four continents and construct broad profiles of their grass flora. We use endemism to examine global patterns of specialisation, and inter-continental distributions as indicators of dispersal, using databases constructed over twenty years. We examined Australasian patterns with regard to endemicity and shared groups and categorised all of the region's genera into four age classes, from Australia's separation from Gondwana to the present. Globally, each continent presented a unique profile and C4 grasses were more widely shared than C3. Australasia's grasses equally comprise C3 and C4 genera; it shared two thirds of its C4 types with other continents, whereas C3 types split evenly between shared and endemic. Australasia shared relatively few genera with just one neighbour (7% C3, 13% C4), primarily with EurAsia. Australasian grass genera and species were either endemic or globally widespread, and 88% of C3 and 93% of C4 species were derived from lineages that originated elsewhere. We conclude Southeast Asia was the gateway for dispersal into Australasia, akin to rainforest taxa exchanges which increased from c12 Ma, with about 65% of Australasia's grass genera arriving in the past 3.5 Ma. The strong presence of C4 grasses in Australasia implies they have infiltrated a wide range of ecosystems, many probably occupied by ancient taxa with which they had not co-evolved.
The circumscription of the grass subtribe Calothecinae has undergone several changes since its description. Currently, it comprises Chascolytrum and the recently described genera Laegaardia and Paramochloa. Here we evaluate the circumscription of Calothecinae and the recently proposed infrageneric classification of Chascolytrum based on a phylogeny with more comprehensive taxon and molecular marker sampling than in previous studies. We sampled all Calothecinae genera, all but one Chascolytrum species, two South American Trisetum s.l., and representatives of the subtribes Agrostidinae, Brizinae, Echinopogoninae, Koeleriinae, Phalaridinae, and Torreyochloinae within Poaceae tribe Poeae. We performed Bayesian and Maximum Likelihood analyses of four plastid DNA regions (atpF-atpH, matK, rps16 intron, and trnL-trnF) and two nuclear ribosomal regions (ITS and ETS). Our results revealed that neither Calothecinae nor Chascolytrum is monophyletic, as currently recognized, because Trisetum brasiliense and Trisetum bulbosum are nested within Chascolytrum. We include these two Trisetum species in Calothecinae as incertae sedis. Laegaardia and Paramochloa form a clade that is sister to the Chascolytrum + Trisetum clade, and based on morphological characters, we transfer the former to the new subtribe Paramochloinae. Our Chascolytrum phylogeny is better resolved and supported than in previous studies, and based on these results, we divide Chascolytrum into nine genera, including two new ones: Boldrinia (gen. nov.), Calotheca, Chascolytrum, Erianthecium, Lombardochloa, Microbriza, Poidium, Rhombolytrum, and Rosengurttia (gen. nov.). We provide a key to Calothecinae genera, descriptions of the genera, nomenclatural information, and keys to species of each genus. In addition, six new combinations are proposed.